Copyright 2012 Robert Clark
Curiosity Surveys a Martian 'Mojave Desert': Big Pic.
Aug. 8, 2012 --
http://news.discovery.com/space/big-pic-mars-curiosity-mojave-desert-120808.html
The panoramic image shows what appears to be "haze" at the base of the mountains in the distance in Gale crater. This was predicted prior to landing:
Pink skies, water ice haze in forecast for Curiosity landing.
12:56 PM, Aug 5, 2012
"PASADENA, CALIF. — Expect pink skies with a chance of a water ice haze over Gale Crater Monday when NASA’s Mars Science Laboratory and Curiosity rover arrive at the red planet.
"Seasonal winter temperatures are expected to be a balmy 10 degrees Fahrenheit when Curiosity touches down at 3 p.m. local Mars time."
http://www.floridatoday.com/article/20120804/SPACE/120804008/Pink-skies-water-ice-haze-forecast-Curiosity-landing
It is important to realize that clouds, fogs and hazes can have some proportion of liquid water even well below freezing temperature. This is well known to happen when salts are dissolved in the water through freezing point depression. But it can also happen with pure water through
supercooling.
The temperature at which supercooled liquid water can occur can even be below -40C, which, coincidentally is also -40F:
Supercool Water.
Posted: 11/28/11
"Liquid water as cold as minus 40 F has been found in clouds. Scientists have done experiments showing liquid water can exist at least down to minus 42 F."
http://www.astrobio.net/pressrelease/4363/supercool-water
**************************************************************
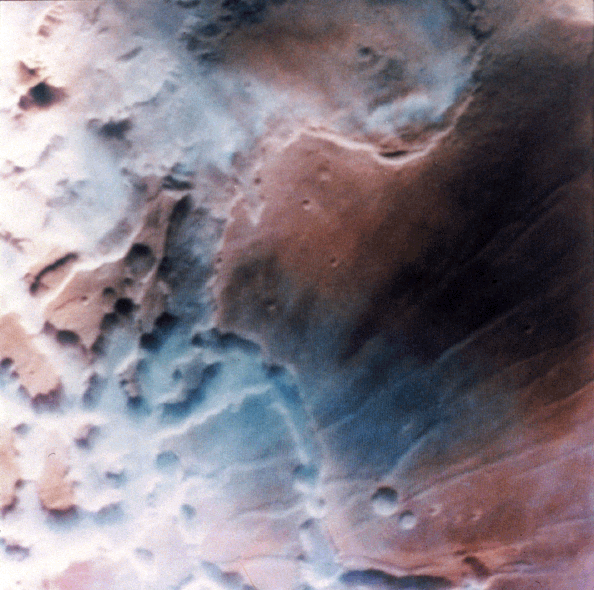
Noctis Labyrinthus, part of the Valles Marineris system, frequently shows dense low lying clouds/fogs that give the appearance of precipitation carrying clouds on Earth:
Clouds in Noctis Labyrinthis.
Credit: NASA, Viking orbiter image.
This image shows early morning fog in the Noctis Labyrinthis, at the westernmost end of Valles Marineris. This fog, which is probably composed of water ice, is confined primarily to the low-lying troughs, but occasionally extends over the adjacent plateau. The region shown is about 300 kilometers (186 miles) across.
http://www.solarviews.com/cap/mars/noctis.htm
Noctis Labyrinthus, labyrinth of the night.
Mars Express
European Space Agency
30 November 2007
http://www.esa.int/SPECIALS/Mars_Express/SEMWBK73R8F_0.html
Here's another great image showing dense clouds/fogs in Valles Marineris somewhat further west of Noctis:
taken from this ESA report:
Adsorption water driven processes on Mars.
D. Möhlmann
FIRST MARS EXPRESS SCIENCE CONFERENCE
21-25 February 2005, ESA/ESTEC
http://sci.esa.int/science-e/www/object/doc.cfm?fobjectid=36779
The author reaches these conclusions:
Adsorption water in the upper martian surface is an actual challenge
to martian surface chemistry and possibly also to exobiology:
* Adsorption water makes possible and/or supports a martian surface
chemistry, also at present: These processes are energetically driven
by photons (UV). Current martian surface chemistry is mainly (non-
thermal) photo-chemistry.
* Existing iron oxides (as haematite), UV and adsorption water are a
cause for the production of oxidizing OH-radicals, which are expected
to contribute to the oxidation of organics (Methane, carbonaceous
meteorites).
* Adsorption water mobilizes acids (as sulfuric acid), which can
modify earlier formed carbonates (surface cover by sulfates, e.g.).
* Adsorption water covered catalytic surfaces of minerals are expected
to be essential agents in non-thermal photo-chemical processes. Photon
driven non-thermal redox-processes on catalytic surfaces might
together with atmospheric CO2 cause a non-biogenic production of
organics (?). Related experiments are in preparation.
* Adsorption water deposits also on the surfaces (cell walls) of
microbes etc. There, it can be a source of water for the microbial
metabolism. Physico-chemical processes can be supported by adsorption
water. To study the relevance of adsorption water for life-processes
is a current challenge to exobiology. Related experiments are in
preparation.
This Mars Express image of Valles Marineris with the dense fogs was taken May 25, 2004 in mid southern Autumn on Mars at a time approaching Mars aphelion.
Equatorial clouds are known to be seasonal on Mars, frequently occurring near aphelion. It is now nearing the end of southern Winter on Mars, at the time of the Curiosity landing. It would be interesting to find out if higher resolution imaging by Mars Reconnaissance Orbiter also detects these dense low lying clouds/fogs during the next southern Autumn on Mars.
Some MRO images near the location of the image with the dense clouds/fogs:
HiRISE | Latitude/Longitude Search Results.
Search by latitude and longitude range.
Latitude from: -25 to -5
Longitude from: 290 to 310 (Note: this is measured in east longitude.)
http://hirise.lpl.arizona.edu/geographikos.php?q1=-25&q2=-5&q3=290&q4=310&order=release_date&submit=Search
**************************************************************
This report suggests clouds may be harder to form on Mars than thought previously:
RELEASE: 07_89AR
NASA Study Reveals Less Water in Mars' Clouds.
Dec. 6, 2007
MOFFETT FIELD, Calif. – Martian clouds may contain less water than previously thought, according to a new NASA study.
New NASA laboratory measurements of simulated martian clouds reveal that scientists may have been overestimating the amount of water in the planet's atmosphere.
"The martian clouds we are studying are composed of water ice, like some clouds on Earth. However, they are forming at very cold temperatures, often below minus 100 degrees Celsius (minus 148 degrees Fahrenheit)," said Tony Colaprete, a planetary scientist at NASA's Ames Research Center, Moffett Field, Calif. "What we have found in our laboratory studies is that it is much harder to initiate cloud formation at these cloud temperatures than what we thought," he explained.
http://www.nasa.gov/centers/ames/news/releases/2007/07_89AR.html
The last statement in the NASA news release is misleading:
The amount of water in the martian atmosphere varies greatly in spaceand time," Colaprete observed. Clouds in the atmosphere largelycontrol the amount of water that comes off of the north pole andmigrates to the south pole."If all the water in the atmosphere were to freeze out to the surface,it would make a layer of ice about one-fifth the thickness of a humanhair, according to Colaprete."Cloud mass is typically only 10 to 20 percent of the total watercontent. However, the thin martian atmosphere is much more sensitive/reactive to the influence of these clouds," he said.
Since the water vapor content on Mars is known to be so low that implies that the water content in any cloud must be even lower. But actually it is because overall the cloud cover of the entire planet is relatively low.
But the water content in precipitation clouds can be much higher than the water vapor content in the surrounding area. For instance during a storm you can have many inches of rainfall or snowfall. But the water vapor content on Earth is at most 5 to 6 precipitable cm, about 2 to 2.5 inches (the amount of water vapor in an atmospheric column if it were condensed.)
The NASA report focused on clouds at very cold temperatures -100C. But it is known there are daytime clouds/fogs very close to the surface on Mars where the temperatures will be much higher than this, frequently above -40C for instance. As mentioned above, this is a temperature at which even pure water in clouds can undergo supercooling to remain in liquid form. Indeed supercooling is a major part of cloud formation on Earth:
Cloud.
http://en.wikipedia.org/wiki/Cloud
In any case clouds at such low temperatures have been observed on Mars. Also even at such low temperatures it is still possible some proportion of the condensed water in the clouds is in liquid form. For instance actual measurements of Polar Stratospheric Clouds on Earth show that liquid water aerosols with nitric and sulfuric acid can be liquid down to -80 C:
Polar Stratospheric Clouds.
Type I a (Nitric acid trihydrate particle - NAT)
crystalline particles forming at 195 K,
Type I b (Supercooled ternary solution - STS)
spherical liquid particles forming at 193 K,
Type II (Water ice) ice crystals forming below 188 K.
Chemical Analysis of Polar Stratospheric Cloud Particles.
Jochen Schreiner, Christiane Voigt, Andreas Kohlmann, Frank Arnold, Konrad Mauersberger, Niels Larsen
Science, 12 February 1999: vol. 283 no. 5404 pp. 968-970.
A balloon-borne gondola carrying a particle analysis system, a backscatter sonde, and pressure and temperature sensors was launched from Kiruna, Sweden, on 25 January 1998. Measurements within polar stratospheric cloud layers inside the Arctic polar vortex show a close correlation between large backscatter ratios and enhanced particle-related water and nitric acid signals at low temperatures. Periodic structures in the data indicate the presence of lee waves. The H2O/HNO3 molar ratios are consistently found to be above 10 at atmospheric temperatures between 189 and 192 kelvin. Such high ratios indicate ternary solution particles of H2O, HNO3 [nitric acid], and H2SO4 [sulfuric acid] rather than the presence of solid hydrates.
http://www.sciencemag.org/content/283/5404/968.abstract
Because these Earth clouds are stratospheric, they occur at pressures near those on the surface of Mars. Then low lying fogs or clouds on Mars would occur at similar pressures and temperatures to the liquid water containing PSC's on Earth.
Also recent work by Bogdan et.al. suggests some liquid water in clouds could remain down to -140C(!)
New Observations On Properties Of Water.
ScienceDaily (Dec. 13, 2006) -- Recent research on the properties of
water reveals information relevant for cloud physics and even
cryopreservation science.
Experimental studies conducted by Ph.D. Anatoli Bogdan at the
University of Helsinki, Finland, have received broad interest in the
scientific world, as the results might have applications even in the
cryopreservation of cells and tissues. Bogdan's results show that
mixture droplets consisting of sulphuric acid and water can be slowly
cooled down to-140 degrees Celsius and then heated again without ice
formation.
http://www.sciencedaily.com/releases/2006/12/061213104104.htm
Reversible Formation of Glassy Water in Slowly Cooling Diluted Drops.
J. Phys. Chem. B, 110 (25), 12205 -12206, 2006. 10.1021/jp062464a S1520-6106(06)02464-3
Web Release Date: June 6, 2006
http://pubs.acs.org/cgi-bin/abstract.cgi/jpcbfk/2006/110/i25/abs/jp062464a.html
This might make it possible for even lower temperature Polar Mesopheric Clouds, also called noctilucent clouds, on Earth to contain liquid water. The Aeronomy of Ice in the Mesosphere (AIM) satellite was recently launched to study such clouds on Earth. If such clouds are also found to contain some proportion of liquid water, that would greatly increase the range of possibilities for liquid water in clouds on Mars.
The term "noctilucent clouds" comes from the fact they often have a luminous appearance at night:
The secrets of night shining clouds.
http://earthsky.org/earth/nasa-satellite-observations-of-noctilucent-clouds-show-complex-atmospheric-interactions
Meteor Smoke Makes Strange Clouds.
August 7, 2012: Anyone who's ever seen a noctilucent cloud or “NLC” would agree: They look alien. The electric-blue ripples and pale tendrils of NLCs reaching across the night sky resemble something from another world.
http://science.nasa.gov/science-news/science-at-nasa/2012/07aug_meteorsmoke/
The NLC's often have a bluish tint. Interestingly it has often been noticed both from ground-based observations and from Mars spacecraft that there are frequent bluish clouds on Mars:
Mars Pathfinder.
Dr. Mark Lemmon, University of Arizona
Mars Pathfinder Imaging Team
These clouds from Sol 15 have a new look. As water ice clouds cover the sky, the sky takes on a more bluish cast. This is because small particles (perhaps a tenth the size of the Martian dust, or one-thousandth the thickness of a human hair) are bright in blue light, but almost invisible in red light. Thus, scientists expect that the ice particles in the clouds are very small. The clouds were imaged by the Imager for Mars Pathfinder (IMP).
http://mars.jpl.nasa.gov/MPF/science/clouds.html
It would be interesting to find out if the NLC's on Earth contain the sulfuric acid content to allow the NLC cloud particles to remain partially liquid down to the extremely low temperatures suggested by Bogdan et.al. It would also be interesting to find out if the bluish clouds on Mars also have this sulfuric acid component.
Bob Clark